4 Diels–Alder Cycloaddition
Adapted from: “Introduction to Organic Laboratory Techniques: A Small Scale Approach” by Donald L. Pavia,
Gary M. Lampman, George S. Kriz and Randall Engel, 3rd Edition, Brooks/Cole Cengage Learning, 2011. pp. 320-
324.
Introduction
Background
The Diels–Alder reaction is an incredibly useful reaction named (in the tradition of many important organic reactions) after its discoverers, Otto Diels and Kurt Alder. In a Diels–Alder reaction, two C–C sigma bonds are formed in a single step without the formation of any intermediates to yield a product with a 6-atom ring. The reaction is further classified as a cycloaddition because it forms a cyclic product, as show in the reaction below of 1,3-butadiene with a substituted ethylene below.
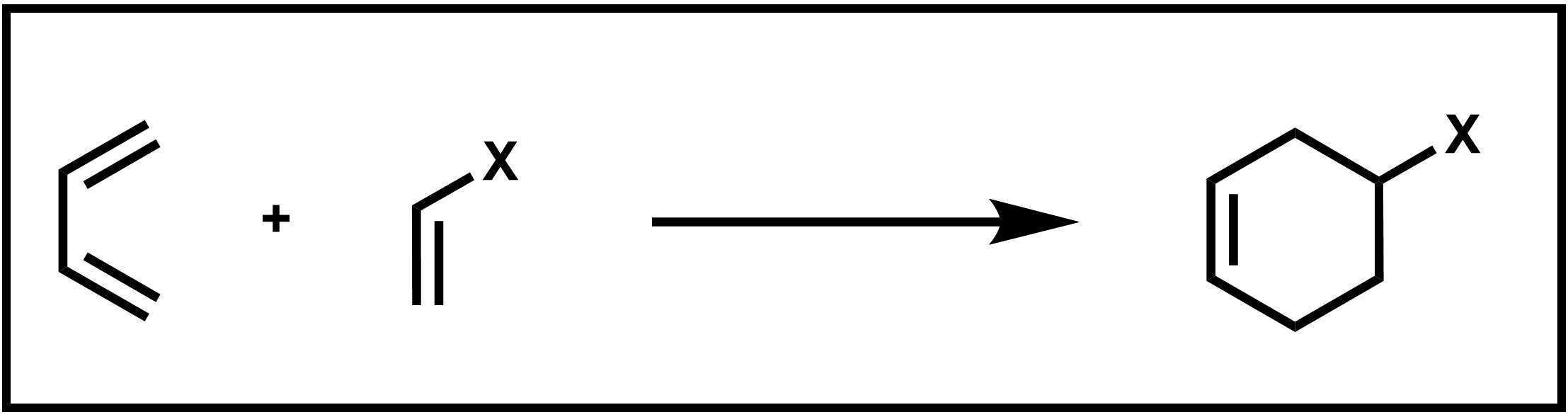
Scheme 1. Diels–Alder addition of a diene and dienophile
Specifically, the reaction is a [4+2] cycloaddition because the reaction occurs between the π systems of two species, one a 4-atom π system which is the diene (1,3-butadiene in the reaction shown above) and the other a 2-atom π system called the dienophile (substituted ethylene in the example reaction).
The cyclic product of this Diels–Alder reaction is a substituted cyclohexene ring. Because this reaction occurs in a single step, without the formation of any intermediates, it can be further described as a pericyclic reaction. A pericyclic reaction has a mechanism that involves several simultaneous bond-making and breaking processes which occur via a cyclic transition state, or otherwise known as a concerted mechanism.
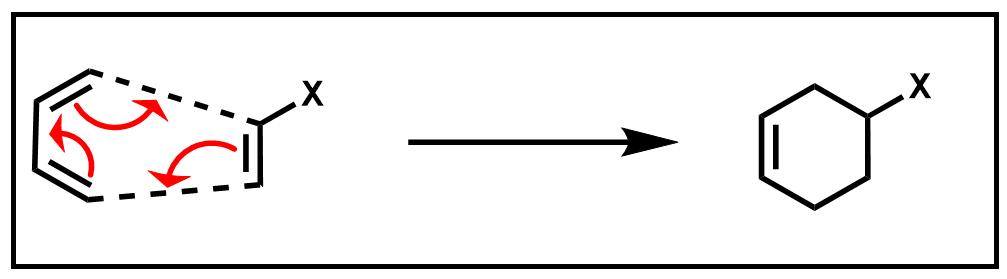
Scheme 2. Concerted mechanism of Diels–Alder reaction
The reactants in a Diels–Alder reaction are a conjugated diene and a dienophile. The example diene, 1,3-butadiene, exists in an equilibrium between an s-cis and an s-trans conformation.
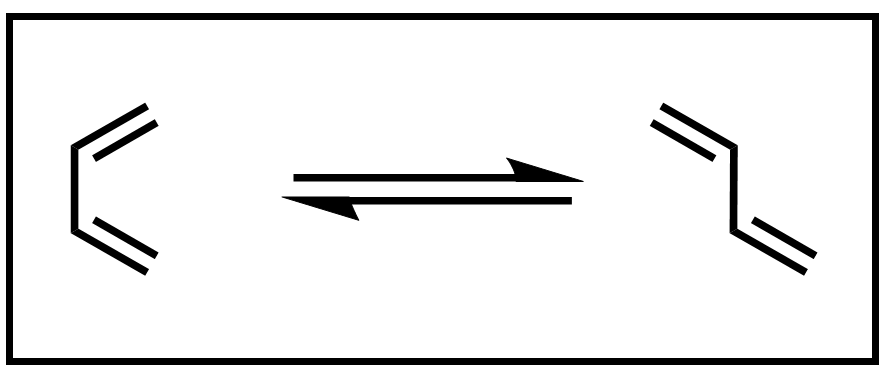
Scheme 3. Equilibrium of 1,3-butadiene conformers
A Diels–Alder reaction can only occur when the diene is in the s-cis conformation. Thus, dienes that are incapable of adopting a s-cis conformation are unreactive in a Diels–Alder reaction.
Dienes such as cyclopentadiene, which you will be working with in this experiment, have the benefit of being permanently locked in an s-cis conformation and therefore are highly reactive in a Diels–Alder reaction. In fact, cyclopentadiene is so reactive that it reacts with itself to form a dimer called dicyclopentadiene.
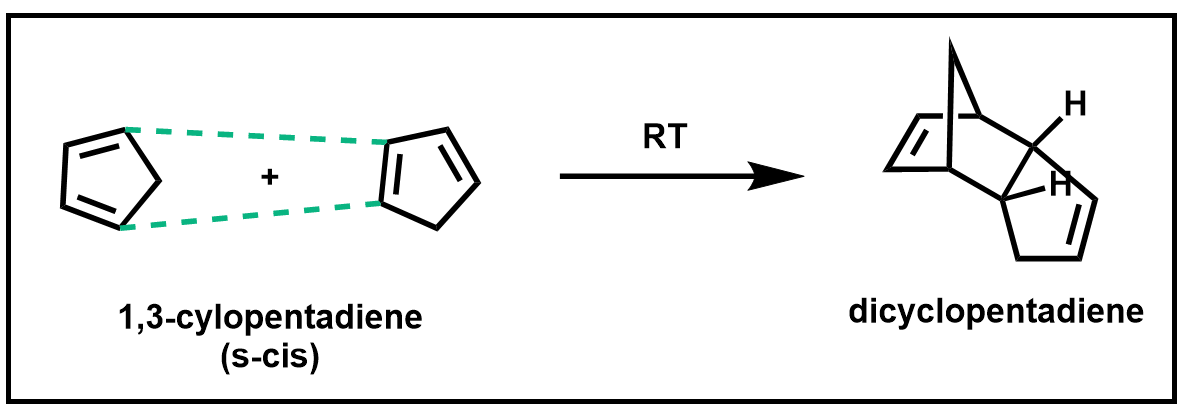
Scheme 4. Diels–Alder of 1,3-cyclopentadiene
When cyclopentadiene is stored at room temperature, it is completely converted into the dimer within a few hours. Therefore, when cyclopentadiene is to be used in a Diels–Alder reaction, it must first be formed from its dimer via a retro Diels–Alder reaction and used immediately or stored at very low temperatures.
With respect to the dienophile, Diels–Alder reactions work best when it is electron deficient. When the dienophile does not contain any substituents (such as ethylene) the reaction proceeds slowly due to a large activation energy. Such reactions cannot easily be heated to overcome because this favors the reactants rather than the cycloadduct product.
An alkyne can also function as a dienophile, with one set of π bond electrons of the alkyne participating in the reaction and the other giving a second π bond in the product.
In this experiment, a retro Diels–Alder reaction will be performed to prepare fresh cyclopentadiene from dicyclopentadiene. You will then immediately use it to run a Diels–Αlder reaction with maleic anhydride.
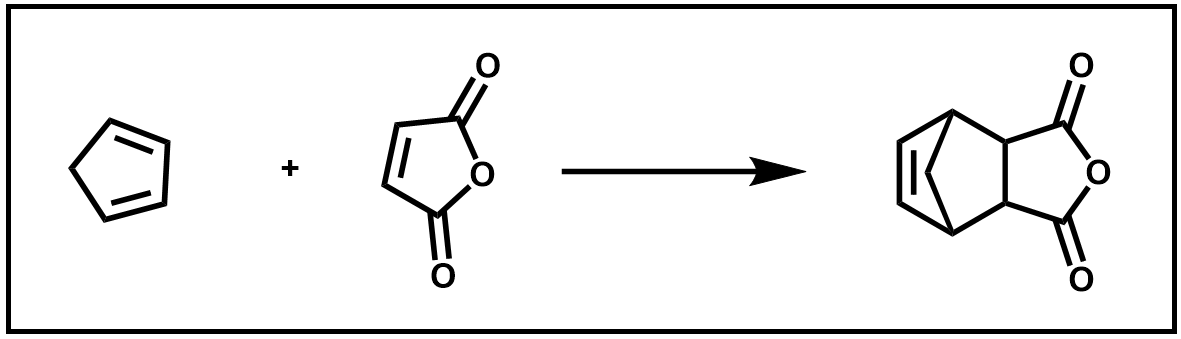
Scheme 5. Diels–Alder of 1,3-cyclopentadiene and maleic acid
Safety & Waste
1,3-cyclopentadiene is a mildly toxic volatile substance with a very foul smelling odor. Its dimer also has a similar foul smelling odor. Hence, these chemicals should be kept inside the fume hoods unless contained within a closed container.
All joints in the distillation apparatus should be connected and secured with Keck clips.
1,3-cyclopentadiene and all other solvents used in this experiment are highly flammable and hence distillation should not be run to dryness.
All glassware that comes in contact with cyclopentadiene and its dimer must be rinsed in the hood with acetone and the rinse must be collected in a beaker BEFORE taking the glassware out of the hood. Dispose of the rinse in the non-halogenated waste container.
All other solvents can be discarded into the appropriate designated waste container(s) in the hood.
Protocol
Formation of Cyclopentadiene
Measure 51.9 mmol of dicyclopentadiene into a 25-mL round bottom flask charged with a stirbar. Attach the flask to a fractional distillation apparatus using a Vigreux column as your fractionating column. Use a small pear-shaped flask as the receiving flask and cool the receiving flask in an ice-water bath. Insulate the Vigreux column with loosely wrapped aluminum foil.
Heat the dimer while stirring (put your stir plate under the heating mantle) until the solution is briskly refluxing. The monomer will distill at 40–42 °C. Distill the monomer as rapidly as possible, but do not exceed a distillation temperature of 43–45 °C. On average, 2.5 mL cyclopentadiene should be obtained from the distillation within 30 minutes. Heating longer than 30 minutes does not typically yield additional distillate, as other reactions may occur. Students who distill more than the amount needed for the next step should keep their distillate cold in the ice-water bath until everyone has sufficient 1,3-cyclopentadiene to proceed.
While the distillation is in progress, measure 15.3 mmol of maleic anhydride and dissolve in 5 mL of ethyl acetate in a 25-mL Erlenmeyer flask. It may be necessary to slightly warm this solution to completely dissolve the maleic anhydride. Once a solution is achieved, it should be cooled in an ice-water bath. Add a small stir bar to this flask.
Cycloaddition and Isolation
Add 1.194 equiv. of freshly distilled 1,3-cyclopentadiene in one portion to the cooled solution of maleic anhydride. Stir the reaction on your stir plate until the exothermic reaction subsides. Add 5 mL of petroleum ether (bp 60–80 °C) to cause the cycloadduct to precipitate as a white solid. Then, heat the mixture on a hot plate until the solid has redissolved.
To prevent the product from oiling out of solution, and to form larger crystals of higher purity, the product must cool slowly. To accomplish this, place the Erlenmeyer flask in a warm 50–60 °C water bath and let the bath and flask slowly cool to room temperature. Once the solution is cooled and no more crystallization is apparent, cool the flask in an ice-water bath for 5 minutes to complete the process. Isolate the product by vacuum filtration, rinsing the ice cold petroleum ether and let the product dry in your drawer until the next lab period.
Characterization
Determine the percent yield of your reaction and characterize your purified by product by melting point, IR, 1H and 13C NMR.
Cleanup
The remaining undistilled dicyclopentadiene and the mother liquors from the filtrations should be disposed of in the non-halogenated waste container in the hood.
Used filter paper should be discarded into the solid waste container and used pipets, melting point capillaries, and other disposable glass should be discarded into the glass waste container.
All glassware that comes into contact with cyclopentadiene and its dimer (i e. the entire distillation setup) must be rinsed the hood with acetone with the rinse collected in a beaker BEFORE taking the glassware out of the hood. This rinse must be dispensed into the non-halogenated waste in the hood. The product should be discarded in the solid waste container after fully characterized.
Wipe down your work area(s) with a damp paper towel and discard the paper towel in the solid waste container prior to leaving the lab.
A glass condenser containing an abundance of downward-pointing indentations, which dramatically increase the surface area per unit length of the condenser. A few types of Vigreux columns have a side arm which allows you to connect a straight condenser directly to the column, allowing you to shorten the height of the fractional distillation setup. Special variations also have a vacuum adapter or may include an outer glass cylinder (jacket), allowing air/fluid circulation or, to aid in insulation, an outer vacuum jacket.
The process of heating the chemical reaction for a specific amount of time while continually cooling the vapor produced back into liquid form using a condenser. The vapors produced above the reaction continually undergo condensation, returning to the flask as a condensate. This way, it guarantees that the temperature of the reaction remains constant.
