2 Chromatography
Background Reading
Please read the original flash column chromatography report after you read this protocol but before lab. You must be on campus (or VPN) to access the paper.
Introduction
Background
In previous lab courses, you may have used thin-layer chromatography (TLC) for presumptive identification of compounds and/or to monitor the progress of a reaction. These uses are typically done a small scale (<1 mg material) so are termed analytical TLC—Meaning, you are using TLC to analyze a compound or mixture of compounds. Though very useful for analysis, analytical TLC is not practical for larger scale separations. However, the same principles can be applied on moderate or large(ish) scale using two similar techniques: Preparatory (prep) TLC and flash column chromatography.
Analytical TLC (commonly abbreviated to just “TLC”) and prep TLC are similar in that they both use a stationary phase coated on a plate and a solvent (eluent) which travels up the plate to separate the components in a mixture. They different in purpose and output of analysis. TLC is primarily used as a qualitative analytical tool that allows for presumptive identification of compounds in a mixture by comparing the distance each compound travels up the plate (Rf) with each other and known standards. In contrast, prep TLC is used to purify and isolate individual components of a mixture on a small scale (typically <100 mg).
In practical terms, there are only a few differences. In TLC, the sample is applied near the bottom of the plate (at the baseline) as a single, small spot; in prep TLC, the sample is applied as a narrow line across the baseline (Figure 1). In both techniques, the compounds are visualized—typically using UV light or TLC stain—after the solvent has run up the plate. The separated compounds can sometimes be presumptively identified at this point, which is where a typical TLC would end. In prep TLC, however, the separated compounds (along with the stationary phase) are scraped off the plate, collected, and the compound is extracted from the stationary phase. The goal is to obtain relatively pure compounds for further analysis or for use in a subsequent synthetic step.

Figure 1. A schematic depiction of analytical versus preparative TLC
Flash column chromatography is a technique used to separate and purify individual components of a mixture. It can be used on large(ish) scale (even kilogram scale, depending on the mixture). In this course, we will regularly use flash columns (commonly abbreviated as “columns”) to purify our material. In column chromatography, a stationary phase—typically silica gel, though sometimes alumina—is packed into a vertical glass column. After applying the mixture to the top of the stationary phase, the liquid mobile phase is added to the top and flows down through the column (Figure 2). “Flash” column chromatography is characterized by the use of air pressure as a means to force the mobile phase (and compounds) down and through the column. Each component in the mixture will interact differently with the stationary and mobile phases; as such, they will be carried down and drip out of the column at different rates, which allows for efficient separation.

Figure 2. A schematic description of analytic TLC versus flash column chromatography
For TLC or Prep TLC, it is common to use only a single solvent (whether one solvent or a mixture of solvents at a set ratio) to develop the plate. However, with column chromatography it is possible to use different solvents. For example, you might use 100 mL of a non-polar solvent such as hexanes (hex), followed by 100 mL of a more polar solvent such as ethyl acetate (EtOAc). You could also gradually increase the polarity of the solvent using a gradient of smaller volumes of solvent with a gradually increasing amount of polar solvent (e.g., 50 mL hex, then 50 mL 20% EtOAc in hex, followed by 50 mL 50% EtOAc in hex, and then finally 100% EtOAc) to improve the separation.
There are also several types of stationary phases that can be used in flash column, preparatory TLC, and TLC, though silica gel (SiO2) is the most common. Silica gel is sensitive to the amount of water which is bound to it: The higher the water content, or the adsorbent, the fewer polar sites it has available to bind organic compounds, and the less “sticky” it is. Moreover, these adsorbents are available in different particle sizes called mesh sizes (e.g., Silica Gel 60). The number identifies the number of holes in the mesh of the sieve used to size the silica in the manufacturing process. The more holes per unit area, the smaller the holes are, and the smaller the silica particles which pass through that mesh. Adsorbent particle size (mesh size) affects how the solvent flows through the column. The best stationary phase (or adsorbent) and mobile phase (or eluent) to effectively separate a mixture a compounds is highly dependent on the precise compound mixture, but can be determined through trial and error.
Experiment Overview
You will separate a mixture of fluorene and fluorenone (~50:50) by prep TLC and flash column chromatography. In both cases, you will use silica gel as the stationary phase and EtOAc/hex as the mobile phase. It is your responsibility to determine the relative amounts of the two mobile-phase solvents.
Safety and Waste
As always, appropriate lab attire and PPE are expected. Goggles must be worn any time chemicals or glassware are out and in use anywhere in the lab.
The solvents are flammable and the silica gel is an abrasive. These chemical must be handled in the fume hood. Note that silica gel is a fine, respirable dust. It can and will cause respiratory tract irritation, including coughing, wheezing, difficulty breathing, and impaired lung function. Use with care.
Do not stare directly into the UV lamp as prolonged exposure can damage the corneas.
General Instructions
Though you should work with others to determine the ideal mobile-phase solvent system, you will run the prep TLC and flash column independently. You will do one part each day and should be prepared to do either on day 1.
Experimental Protocol
Part I. Mobile Phase Optimization
You will be given EtOAc and hexanes for your mobile phase. It is your responsibility to determine the ideal mobile-phase composition using analytical TLC. You can (and should) work in groups to make this determination. Each person should use no more than 3 TLC plates for this initial determination. (You have a limited supply throughout the semester!)
Part II. Flash Column Chromatography
Read the original report of flash column chromatography (linked above) before lab!
Protocol Note
You have a glass column in your fume hood. Use the metal rod to push a small cotton plug to the bottom of the column. Sprinkle a small layer (0.5–1 cm) of clean, dry sand atop the cotton. Clamp the column vertically in your hood and close the stopcock. Place a clean, dry beaker under the column. Prepare 100 mL of your chosen solvent system (eluent).
Add some silica gel to a clean, dry 125-mL Erlenmeyer flask. Add some of your eluent to the flask and swirl until the solution moves freely. Add this solution to the column. Rinse the sides of the column with fresh eluent. Open the column stopcock and gently add air pressure to the top of the column to drain the solvent and pack the silica. To avoid drying out the stationary phase, always leave some solvent above the top of the silica bed. Once your solvent line is just above the top of the silica bed, remove the air pressure and close the stopcock. Repeat this process until your silica column is about 5–6 inches tall. Then, drain your solvent to the very top of the silica bed. (Remember, do not let it dry out!) Add another small layer (0.5–1 cm) of clean, dry sand to the top of the silica bed. Use a glass pipette to carefully add fresh eluent to the top of the sand. At this point, the goal is to disturb the top of the silica bed as little as possible. Drain the solvent to the very top of the sand bed.
Dissolve your compound mixture in the minimum amount of fresh eluent. Use a long glass pipette to carefully add the solution to the top of the sand bed. Again, you want to be as gentle as possible so as not to disturb the silica bed. Drain the solution to the very top of the sand bed. Carefully rinse the sides of the glass column with a small amount of fresh eluent. Drain the solution to the very top of the sand bed. Carefully add fresh eluent to the top of the sand bed. Continue to add solvent to just below the ground-glass joint of your column.
Replace the beaker under your column with a test tube. Open the stopcock and use gentle pressure to elute the solvent—And with it, your compounds. Use the original paper to determine the optimal flow rate. Collect fractions by swapping the test tube for a new one as it becomes full. Keep your fractions in order. Keep a close eye on the solvent level so that you do not dry out your silica bed.
Once you have collected about 10–12 fractions, remove the pressure and close the stopcock. Evaluate each fraction by analytical TLC. If a compound is still eluting, add more solvent and collect a few more fractions. Reevaluate by analytical TLC. Once both compounds have fully eluted, collect and combine the clean fractions of compound 1 (either fluorene or fluorenone) into a tared flask. Separately, collect and combine the clean fractions of compound 2 (the other one) into a different tared flask. Concentrate the solvents by rotary evaporation. (Your TA will demonstrate use of the rotary evaporator or “rotavap.”) Leave the uncapped flasks in your drawer until the next lab session. Then, obtain the mass of each purified compound and determine your percent recovery. Obtain an IR spectrum of each to establish the identities.
Part III. Prep TLC
A pictorial description of prep TLC follows.
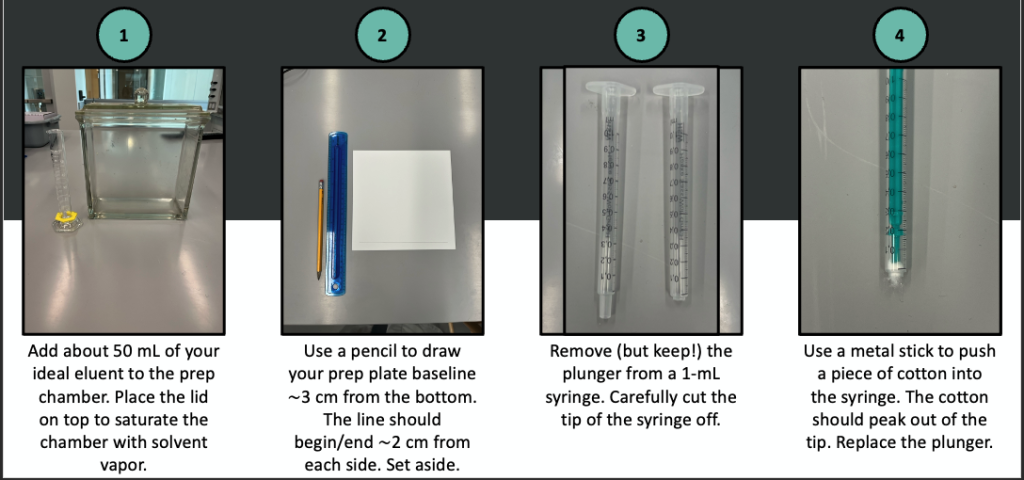

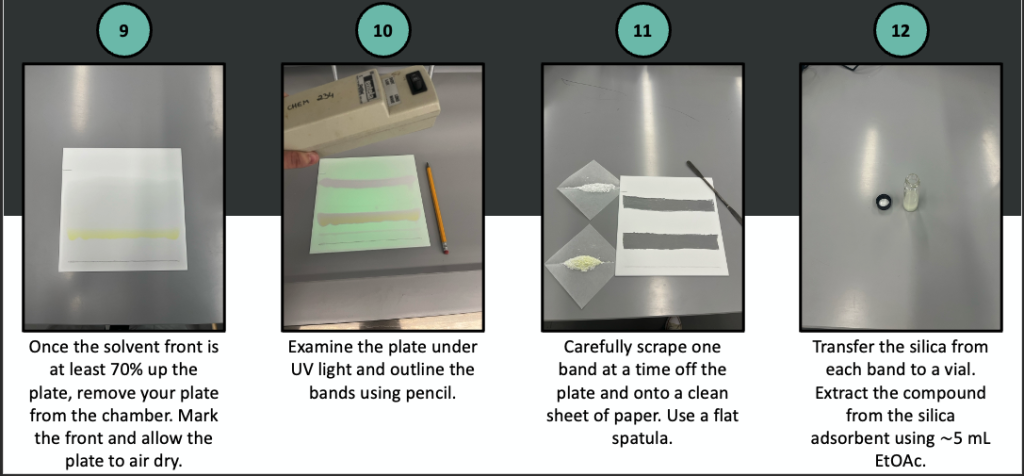
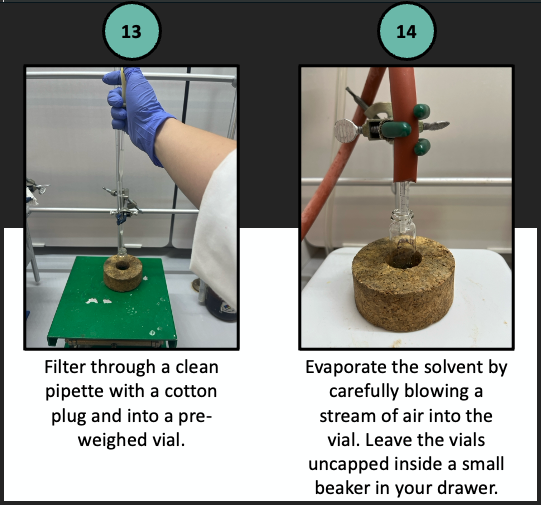
Once dry, measure the mass of each purified compound and determine your percent recovery. Obtain an IR spectrum of each to establish the identities.
Clean-Up
Columns should be drained by opening the stopcock and forcing the solvent through the silica until it runs dry. Dispose of the now-dry (but used) silica in the solid waste container. Rinse the glass column with water, then acetone. Clamp your column in the fume hood upside down with the stopcock open. (Remember, this is your hood but you are sharing it with students in other sections—Please keep it clean!)
Used prep TLC plates should be stacked in the waste fume hood. Any other glass solid waste (e.g., TLC spotters) should be disposed of in the glass waste. All non-glass solid waste items (including the syringe that you used to load your prep plate, TLC plates, etc.) should be disposed of in the solid waste container.
All other glassware should be rinsed with acetone, soap/water, then acetone again. Analytical TLC chambers should not be rinsed; discard the solvent then leave the chamber uncapped in your drawer. Prep TLC chambers should not be rinsed; discard the solvent and leave the chamber uncovered in your fume hood.
