9 Michael Addition and Aldol Reaction
Adapted from: “Introduction to Organic Laboratory Techniques: A Small Scale Approach” by Donald L. Pavia,
Gary M. Lampman, George S. Kriz and Randall Engel, 3rd Edition, Brooks/Cole Cengage Learning, 2011. pp. 320-
324.
Background
Introduction
This experiment illustrates how two important synthetic reactions can be combined to prepare an α,β-unsaturated ketone—in this case, 6-ethoxycarbonyl-3,5-diphenyl-2-cyclohexenone. The first step in our sequence is the sodium-hydroxide-catalyzed conjugate addition of ethyl acetoacetate to trans-chalcone (a Michael addition reaction). Be sure you can draw the curved-arrow mechanism of this transformation!

Scheme 1. Michael Addition Reaction
The second step is a base-catalyzed aldol condensation reaction. The base abstracts the most acidic proton (which is the most acidic?) and the resulting carbanion attacks the carbonyl group. A stable six-membered ring is formed. Ethanol supplies a proton to yield the initial aldol product.
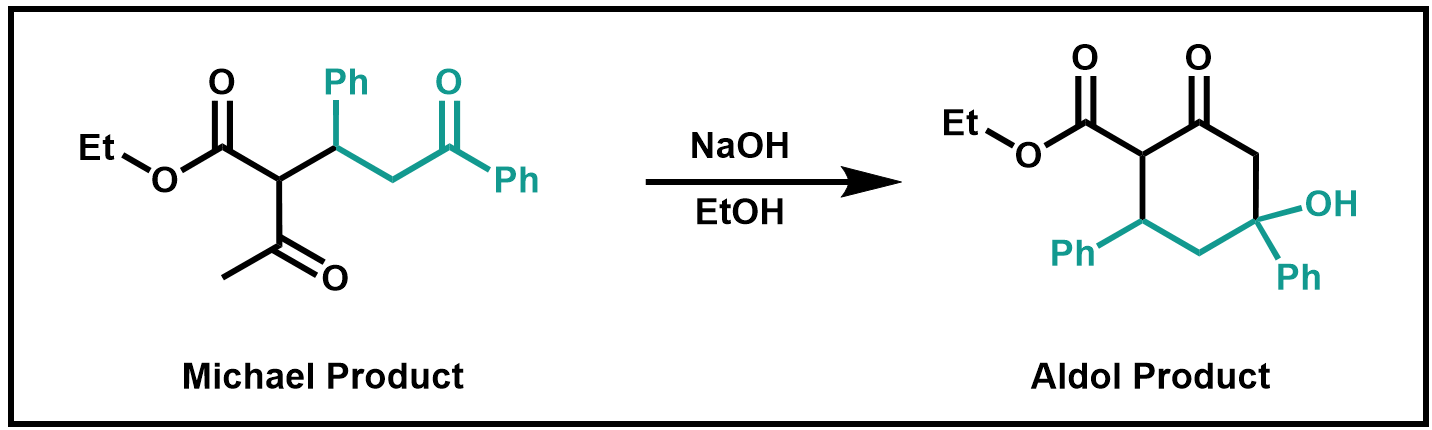
Scheme 2. Aldol Reaction
Finally, the aldol intermediate is dehydrated to form the final product, 6-ethoxycarbonyl-3,5-diphenyl-2-cyclohexenone. The resultant α,β-unsaturated ketone is stabilized by the two flanking π systems. Can you draw at least one resonance form to depict this stabilization?
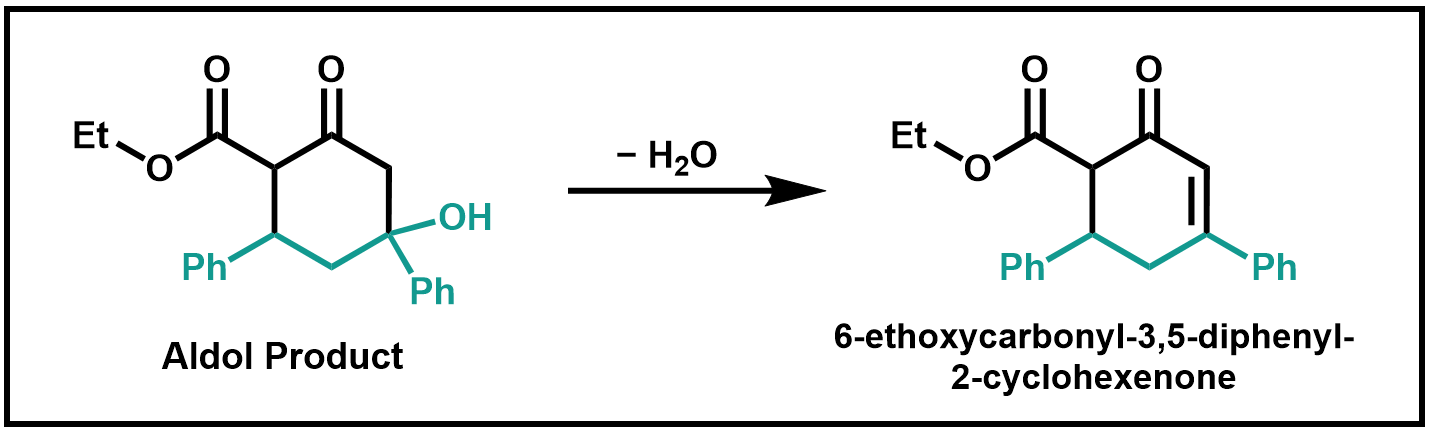
Scheme 3. Dehydration of Aldol Product
Waste Disposal
Dispose of all aqueous wastes containing ethanol in the bottle designated for aqueous wastes. Ethanolic filtrates from crystallization of the product should be poured into the non-halogenated organic waste container.
Experimental Protocol
Reaction Procedure
To a 100-mL round-bottom flask, add 5.76 mmol of finely ground trans-chalcone, 1 molar equiv. of ethyl acetoacetate and 25 mL of 95% ethanol. Stir the flask with a magnetic stir bar to facilitate dissolution of the solid. Add 1 pellet (between 0.090 and 0.120 g) of sodium hydroxide. Weigh the pellet quickly before it begins to absorb water.
Protocol Note
The sodium hydroxide used in this experiment must be kept dry. Be sure to keep the bottle closed when in not in use.
Attach a reflux condenser to the round-bottom flask and heat the mixture under gentle reflux. Use the lowest heat setting that attains a reflux. Once the mixture has been brought to a gentle reflux, continue to reflux for 1 hour. During this time, the mixture will become very cloudy and solid may begin to precipitate. The solution may bump during this process. In other words, the solid in the reaction flask will start to “erupt” and throw solid up into the reflux condenser. You will need to reduce the setting of the heat source to avoid this problem.
Isolation of the Crude Product
After the end of the reflux period, pour the reaction mixture into approximately 15 grams of ice. Stir the mixture while the ice melts and the solution cools. Scratch the inside of the flask with a glass stirring rod to induce crystallization.
Protocol Note
The product may form an oil instead of a crystal during the crystallization process. It is essential to scratch vigorously to prevent this from happening.
Place the flask in an ice bath for a minimum of 30 minutes. It is essential to cool the mixture thoroughly in order to completely crystallize the product. Because the product may precipitate slowly, you should also scratch the inside of the flask occasionally over the 30-minute period in the ice bath.
Collect the precipitate by vacuum filtration. Use 4 mL of ice-cold water to aid in the transfer. Then rinse the round-bottom flask with 3 mL of ice-cold 95% ethanol to complete the transfer of the remaining solid from the flask to the Büchner funnel. Transfer the filter cake to a large piece of filter paper and press out the excess liquid. Allow the solid to air dry overnight. Weigh the dry product.
Purification & Characterization
Prepare a short column of silica gel (~2–3 in) to purify the crude product. Determine the percent yield of your reaction and characterize your purified product by melting point (literature value 111–112), IR, and 1H NMR. The reference spectra are shown below.
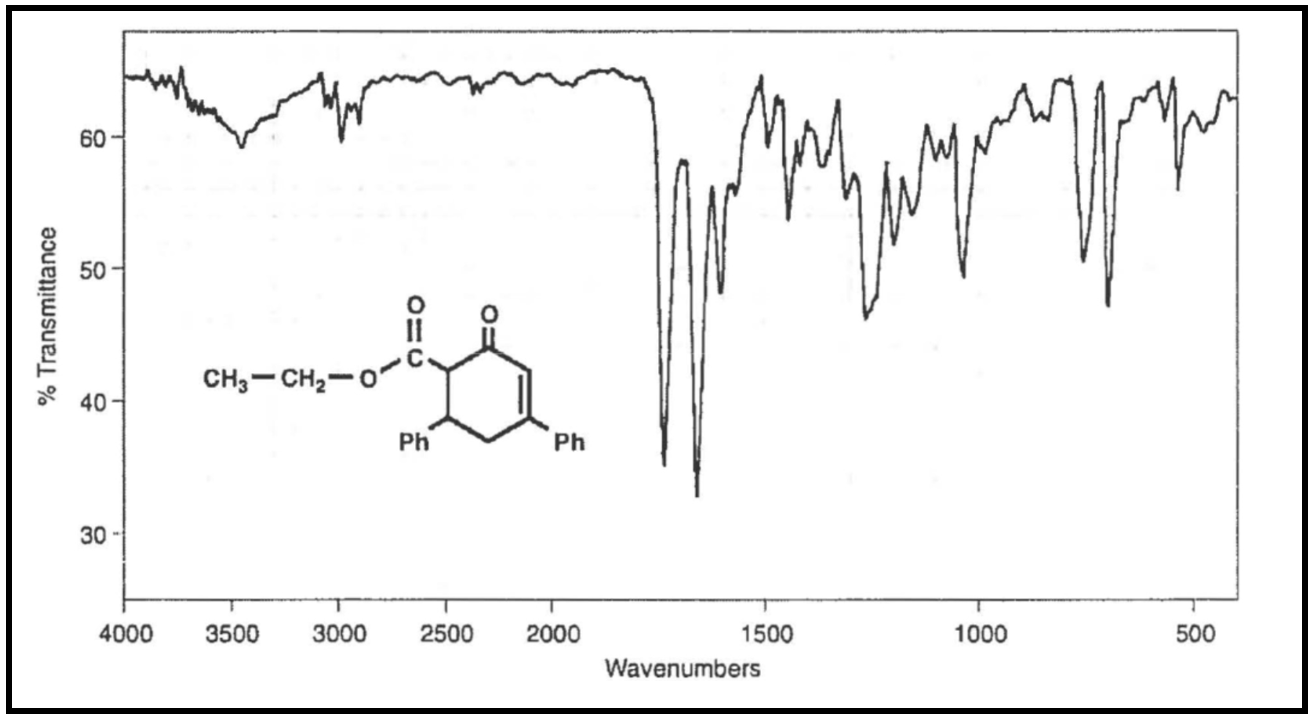
Figure 1. Infrared spectrum of 6-ethoxycarhonyl-3,5-diphenyl-2-cyclohexenone.

