5 Friedel–Crafts Acylation of Anisole
Adapted from: “Introduction to Organic Laboratory Techniques: A Small Scale Approach” by Donald L. Pavia,
Gary M. Lampman, George S. Kriz and Randall Engel, 3rd Edition, Brooks/Cole Cengage Learning, 2011. pp. 508-
514.
Introduction
The Friedel–Crafts reaction is the reaction of an arene (e.g., benzene) with an alkyl or acyl halide (most commonly chloride) using a strong Lewis acid as a catalyst. Anhydrides also engage with arenes under similar conditions. These reactions proceeds via electrophilic aromatic substitution to form an alkylated or acylated arene derivative.
Historically, the first mention of a metal-promoted aromatic acylation appeared in 1973 in a preliminary communication. The authors aimed to produce benzil, but formed benzophenone instead. Following these studies, the preparation of aryl ketones from a reaction of aromatic hydrocarbons and acyl chlorides in the presence of zinc metal or zinc oxide were reported in the same year and in 1876 by different authors. Despite all authors recognizing the presence of zinc chloride in the final reaction mixture, no mention was made of the possible role of a metal halide as a catalyst in the reaction on proceeding papers.
The reaction is named after Charles Friedel of Strasbourg, France and James Mason Crafts of Boston, Massachusetts who developed the reaction successively in 1877. Together, they published a paper that clearly acknowledged the crucial role of the metal halide as a catalyst in the alkylation as well as acylation of aromatic compounds. Additionally, they also studied the generality and the limitations of the new synthetic method. They have shown that the reaction could be successfully applied to a large number of aromatic compounds as well as alkyl chlorides, acyl chlorides, or anhydrides in the presence of chlorides of certain metals such as aluminum, zinc, and iron.
In this reaction, the Friedel–Crafts acylation of anisole (our arene) will be carried out using acetyl chloride in the presence of aluminum chloride (AlCl3), a strong Lewis acid catalyst. The resulting electrophilic aromatic substitution forms only the monoacylated product under these conditions.

Scheme 1. Friedel–Crafts acylation of anisole
Safety & Waste
Both acetyl chloride and aluminum chloride are corrosive reagents. Do not allow them to come in contact with your skin. Take special care not to breathe them in because they generate HCl in the presence of moisture. They may even react violently on contact with water. When working with aluminum chloride, be especially careful to watch out for powdered dust. Weighing and dispensing operations should be carried out in a fume hood when possible. All work, particularly the workup procedure, should be performed in the fume hood.
As usual, place organic liquids in the container for nonhalogenated organic waste unless they contain dichloromethane. Waste solutions that contain dichloromethane should be placed in the container for halogenated organic waste.
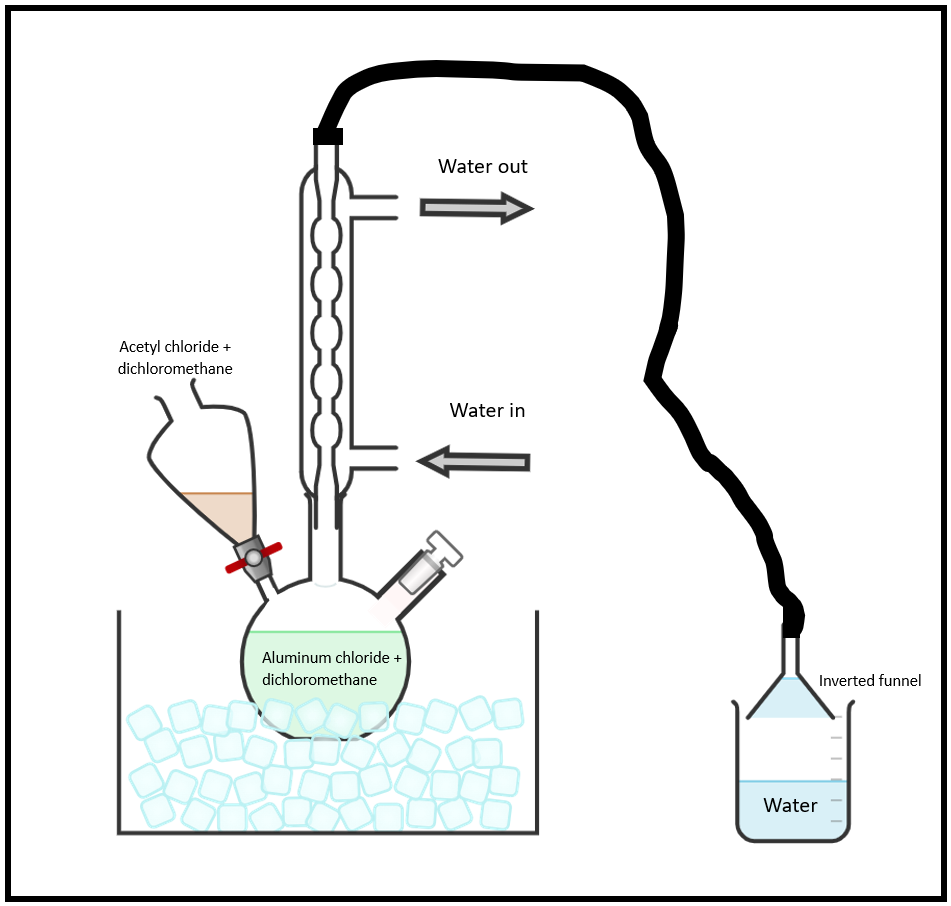
Figure 1. Glassware Setup
Procedure
Experimental Setup
Assemble the reaction apparatus (Figure 1) over your hot plate. All the glassware must be dry because aluminum chloride and acetyl chloride are moisture sensitive. Using a 3-neck, 500-mL round bottom flask, attach a reflux condenser to the central neck and a separatory funnel to either side neck. Use a stopper for the unused third neck. Connect a gas trap as shown to the top of the reflux condenser by attaching rubber tubing from its exit to an inverted funnel placed about 2-mm above the surface of a small volume in a 250-mL beaker. Use a clamp to hold and secure the funnel in place above the water. Place an ice water bath under the 3-necked flask.
Reaction Protocol
Measure 25 mL of dichloromethane in a graduated cylinder. To avoid reacting the aluminum chloride with ambient moisture, measure 105 mmol into a 125-mL beaker in a quick and careful manner. Using a powder funnel and a large spatula, transfer the aluminum chloride into the three-necked flask via the unused opening.
Use the dichloromethane to transfer any last traces of the aluminum chloride powder into the flask and to rinse the neck of the flask. After addition of the dichloromethane is complete, add a stir bar to the flask and replace the stopper. Begin to stir the resulting suspension and start running the cooling water through the condenser.
Using a pipet in a hood, measure out 100 mmol of acetyl chloride into a graduated cylinder and transfer to a small Erlenmeyer flask. Measure 15 mL of dichloromethane in a graduated cylinder and mix with the acetyl chloride. Transfer the resulting solution to the addition funnel attached to the reaction apparatus. Over approximately 15 minutes, slowly add the acetyl chloride solution to the aluminum chloride suspension in a dropwise manner.
After this addition is complete, dissolve 0.75 equiv. of anisole (relative to acetyl chloride) in 10 mL of dichloromethane. Transfer this solution to the same addition funnel and slowly add it to the cooled acylation mixture dropwise over approximately 30 minutes. After this initial 30 minute period, remove the ice water bath and allow the reaction to further stir at room temperature for an additional 30 minutes.
Isolation of Product
Carefully disconnect the gas trap, condenser, and addition funnel. Pour the reaction mixture into a 400-mL beaker containing 50 g ice and 25 mL concentrated hydrochloric acid. Stir this mixture thoroughly for 10–15 minutes. Using a separatory funnel, separate the organic layer and save it. Extract the aqueous layer with an additional 30 mL of dichloromethane and combine the two organic phases. Wash the combined organic layers with 50 mL saturated sodium bicarbonate solution.
Protocol Note
Be sure to vent the separatory funnel more often than you think you need to. Pressure will build if a significant amount of acid is present in the solution and thus caution should be taken.
Continue mixing and venting until the carbon dioxide emission ceases. Wash with a second portion of sodium bicarbonate if necessary. Rinse the organic layer with 20 mL of brine and further dry it after collection by using granular anhydrous sodium sulfate (10–15 minutes). Decant or filter to remove the drying agent from the solution. If necessary, the solution can be stored in a stoppered flask until the next lab period.
Purification of Product
Concentrate the crude acylation product by removing the dichloromethane through rotary evaporation. Transfer the resulting oil to a tared 25- or 50-mL round bottom flask. Record the amount gathered as a crude yield. Take approximately 100 mg of the product and purify it using flash column chromatography; be sure to note the exact amount. Record the resulting product as your actual yield, noting that it is calculated based on the ~100 mg sample you purified. Characterize the purified product using IR, 1H, and 13C NMR.