7 Wittig Reaction and Isomerization
Procedures adapted from: “Experimental Organic Chemistry: A Miniscale and Miccroscale Approach” by John C.
Gilbert and Stephen F. Martin, 6th Edition, Cengage Learning, 2015. pp. 590-591.
Introduction
Background
Previously, we performed a reduction of a carbonyl compound by reaction with a hydride (reducing) agent to yield an alcohol product. Carbonyl functionalities however are not limited to only reduction reactions; this functional group is susceptible to a large variety of chemical transformations, giving it a significant place as an important synthetic building block. In this experiment, we will examine another type of reaction that occurs by nucleophilic addition to a carbonyl: the Wittig reaction.
The Wittig reaction is named after George Wittig who was awarded the Nobel Prize in Chemistry in 1979 for its discovery. The reaction occurs between an aldehyde or ketone and a phosphorus ylide. An ylide is a neutral molecule which contains a negatively charged atom (a carbon anion) directly attached to a positively charged heteroatom (a phosphorus cation in this case). The phosphorus ylide, or Wittig reagent, adds to the carbonyl to produce an alkene by forming a new carbon–carbon π bond at the location of the carbonyl group such that one of the carbon atoms of the alkene is from the carbonyl compound and the other is from the Wittig reagent.
 Scheme 1. General Wittig reaction
Scheme 1. General Wittig reaction
During this reaction, a key intermediate formed is a four-member ring called oxaphosphetane as shown below. There is some debate whether this intermediate is formed by a 1- or 2-step process, and there is evidence that the mechanism varies based on the reactants and other experimental conditions. This cyclic intermediate then undergoes a fragmentation to yield the alkene and triphenylphosphine oxide.
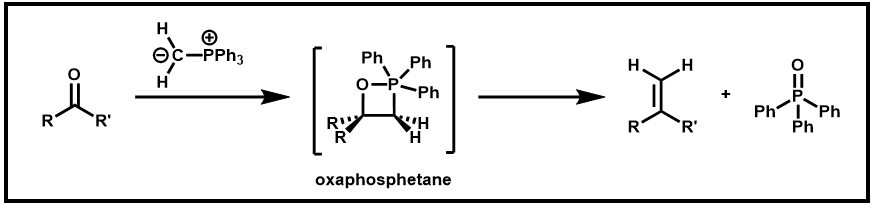 Scheme 2. Formation of oxaphosphetane intermediate
Scheme 2. Formation of oxaphosphetane intermediate
The Wittig reaction and variations upon this reaction, such as the Horner–Wadsworth–Emmons reaction, are important synthetic tools for an organic chemist because they yield a pure alkene of predictable structure. The new alkene group is formed exactly where the carbonyl group was. The E- or Z- geometry of the alkene can vary, although using different types of yildes can control the alkene geometry reasonably well. Investigating the selectivity of the alkene geometry is one of the objectives of this experiment.
Experimental Overview
You are going to prepare stilbene by performing a Wittig reaction. The initial product will be a mixture of isomers, E- and Z-stilbenes, the ratio of which can be determined by integration of the 1H NMR spectrum.
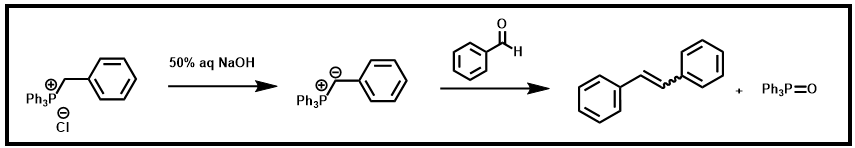 Scheme 3. Synthesis of stilbene
Scheme 3. Synthesis of stilbene
You will then perform an iodine-induced isomerization reaction and determine the new ratio of products.
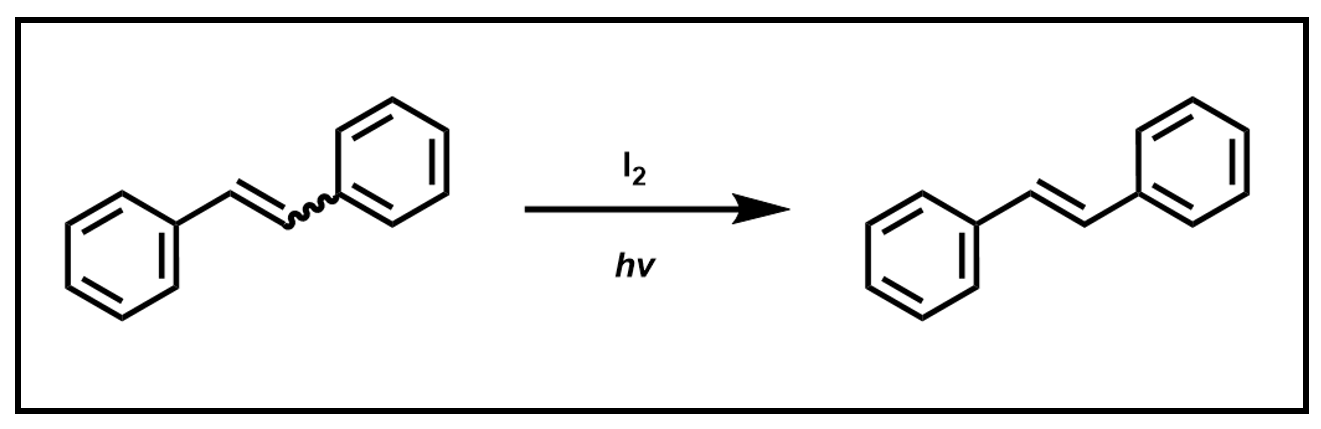 Scheme 4. Isomerization of stilbene
Scheme 4. Isomerization of stilbene
Finally, the product will be purified by recrystallization to yield the most stable isomer in pure form.
Safety
The 50% aqueous solution of sodium hydroxide is highly caustic. Do not allow it to come into contact with your skin. Wash any affected area with dilute acetic acid and then copious amounts of water for 15 minutes. Wear gloves when preparing and handling this solution.
Experimental Protocol
Setting Up Esterification Reaction
Prepare a 50% (by mass) sodium hydroxide solution by dissolving 125 mmol of sodium hydroxide in 5 mL water. This will take some time to dissolve and may require slight heating to facilitate dissolution. If heating was used, the solution should be cooled to room temperature before proceeding.
Add 9.77 mmol of benzyltriphenylphosphonium chloride (caution, toxic) and 9.8 mmol of benzaldehyde (obtained by a micropipette) to a 50-mL round bottom flask containing 10 mL dichloromethane and a stir bar. Equip the flask with a water-cooled reflux condenser and heat the solution while stirring until a gentle reflux is attained.
Reaction
Using a disposable pipet, add the sodium hydroxide solution dropwise through the top of the reflux condenser. Try to add the solution directly into the flask without getting it on the sides of the condenser. The resulting mixture is biphasic and should be stirred as vigorously as possible to ensure complete mixing of the phases. After the addition of base is complete, continue heating the reaction under gentle reflux for 30 minutes.
Workup
Allow the reaction mixture to cool to room temperature and transfer it to a separatory funnel. Rinse the round-bottom flask with a 5-mL portion of dichloromethane and transfer the rinse to the separatory funnel. Separate the phases, collecting the dichloromethane phase in a small Erlenmeyer flask.
Workup Note
Dichloromethane is volatile and likely to evaporate. You may need to add additional dichloromethane to maintain a volume of approximately 15 mL as you proceed with the workup.
Wash the organic phase sequentially with 10 mL water and then 15 mL saturated aqueous sodium bisulfite. Finally, wash the organic phase with 10 mL portions of water until the pH of the water wash is neutral (note this is not the pH of the combined water washes).
Transfer the organic phase to a dry Erlenmeyer flask and dry over anhydrous sodium sulfate.
To determine the ratio of (Z)- and (E)-stilbene in the crude reaction mixture by 1H NMR spectroscopy, remove approximately 0.5 mL of the product solution (use care not to get any drying agent) and place the liquid in a small vial or round bottom flask. Remove the solvent by evaporation.
Store the remaining solution over the drying agent until the next class period.
Isomerization
Filter or decant the remainder of the dry dichloromethane solution into a 25-mL round bottom flask containing a stir bar. Rinse the sodium sulfate with ~2 mL dichloromethane and transfer this rinse into the round bottom flask. Add 0.2955 mmol of molecular iodine to the dichloromethane solution of your stilbene isomers. Equip the flask with an air condenser and irradiate the solution while stirring for 60 minutes with a 150-W lightbulb. You will use one lightbulb per hood.
Safety Note
The 150-W light bulbs are powerful emitters of hazardous UV light. You will need to place a sheet of foil in front of the reaction setup to block the light from your and your classmates’ eyes.
Workup
After the isomerization is complete, decant the reaction solution into a separatory funnel followed by a rinse with an additional 2–3 mL dichloromethane. Wash the solution with a 5 mL portion of saturated aqueous sodium bisulfite and make sure to shake the mixture well prior to allowing the phases to separate. Wash the organic phase with a 5-mL portion of saturated sodium chloride and then transfer it to a dry Erlenmeyer flask and dry over anhydrous sodium sulfate. Once dry, decant the solution into a dry (tared) round bottom flask and remove the solvent by rotary evaporation.
Remove about 50 mg of the crude isomerized product for analysis by 1H NMR spectroscopy.
Purification
Purify the remaining isomerized stilbene product by recrystallization from hot 95% ethanol (this should require no more than 10–12 mL). Cool the hot solution slowly to room temperature and then in an ice-water bath for 15–20 minutes to promote complete crystallization. Collect the precipitate and rinse it with ice cold ethanol, allowing for it to air dry after. Determine the yield of your reaction.
Analysis
You will characterize three distinct products from this reaction. The initial product of the Wittig reaction and the product after the iodine and light-induced isomerization should be characterized only by 1H NMR. The third and final product obtained after recrystallization should be characterized by melting point, IR, and 1H NMR spectroscopy. You will not obtain 13C NMR spectra of any of the products.
Cleanup
All aqueous solutions from the biphasic workups should be disposed of in the appropriate waste containers in the hood. Used drying agents, filter paper, and any other solids should be discarded into the solid waste container.
Working in your hood, rinse all glassware with acetone and collect the rinse in a beaker. Discard this rinse in the non-halogenated waste container. Then wash the glassware with soap and water at the sink followed by an acetone rinse.
Wipe down your work area(s) with a damp paper towel and discard the paper towel in the solid waste container prior to leaving the lab.
