6 Reduction of 4-tert-butylcyclohexanone
Introduction
Background
In general chemistry, you were likely introduced to reduction reactions as electron transfer processes in which an ion or a neutral atom gains electrons On the flip-side of this coin lies oxidation, a reaction in which an ion or neutral atom loses electrons.
In organic chemistry, however, when we describe a reaction as an oxidation or a reduction, we are generally referring to a change in the electron density at carbon. Recall that carbon typically forms covalent bonds; in organic processes, carbon usually goes from neutral species to neutral species. The key change is, therefore, the electron density about that carbon atom, not a change in total electron count. To use the common mnemonic:
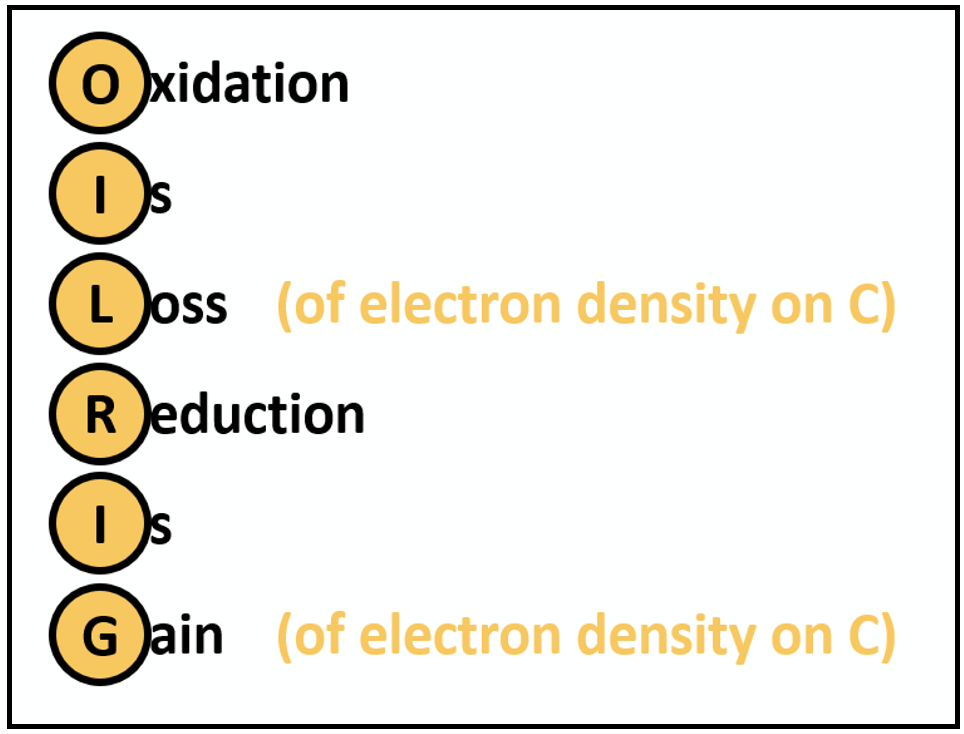
Figure 1. Mnemonic of oxidations and reductions
In practical terms, an oxidation occurs with the loss of C–H bonds or the gain of C–O, C–N, or C–X bonds (X = halide). A reduction occurs with the gain of C–H bonds or cleavage of C–O, C–N, or C–X bonds. What do these bonds have in common?
Among the most common organic redox processes is the interconversion of carbon–oxygen single and double bonds. Carbonyl compounds can be reduced to, for example, alcohols; alcohols can be oxidized to, for example, carbonyl compounds. For this week’s reaction, we will focus in on reduction reactions. Specifically, the reduction of a ketone to a secondary hydroxyl.
In lecture, you learned about the use of metal hydrides to effect this transformation. Two of the simplest metal hydrides used to reduce ketones are sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH or LAH), both of which are reducing agents or reductants—That is, reagents that reduce other compounds. Reductants lie along a spectrum of reducing power and reactivity. NaBH4 is a mild reductant and can be used in alcoholic or even aqueous solutions because it reacts much more rapidly with carbonyl groups than with the solvent. This mild reactivity also leads to some manner of selectivity; NaBH4 reduces ketones and aldehydes, and leaves carboxylic acids, esters, and amides untouched. In contrast, LAH is highly reactive and reacts rapidly (even violently) with protic solvents; it must be used in anhydrous ethereal solvents such as diethyl ether or tetrahydrofuran. LAH will reduce virtually ay carbonyl functional group. There are many other reductants available to the modern organic chemist; the choice of reagent is determined by the particular reaction (and substrate) at hand.
The reduction of a carbonyl to a hydroxyl converts an sp2-hybridized carbon to an sp3 center. Meaning, it is possible to form a new chiral center through this process. If the hydride approaches from either “face” of the carbonyl carbon with equal likelihood, the reduction product of an achiral ketone is a racemic mixture. However, the stereoselective reductions are possible when using a chiral reactant, reductant, or environment. Even the steric bulk of the reducing agent may lend to a favored product and yield a mixture of products enriched in one enantiomer or diastereomer over another.
In this experiment, you will perform the reduction of 4-tert-butrylcyclohexanone using two different reductants: NaBH4 and lithium tri-sec-butylborohydride (L-Selectride). The substrate is achiral, but the reduction produces cis–trans isomers (Scheme 1).
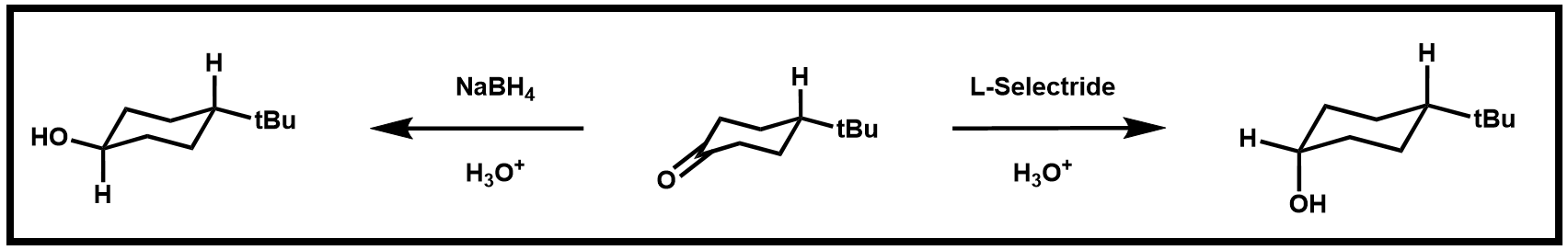 Scheme 1. Reduction to produce cis–trans isomers
Scheme 1. Reduction to produce cis–trans isomers
Reduction by aluminum or boron-derived hydride reagents is irreversible. The stereoselectivity of the reaction depends on the steric bulk of the reducing agent. Small reducing agents (such as NaBH4) predominately approach the carbonyl from the axial side to yield the trans-isomer, while bulkier reagents (such as L-Selectride) deliver the hydride from an equatorial approach to yield the cis-isomer as the major product. You will determine the ratio of the product isomers from each reduction.
The product of these reductions—which contain mixtures of cis- and trans-isomers—have a very complicated 1H NMR spectrum. Nevertheless, the ratio of isomers can be determined through analysis of the hydrogen on the hydroxyl-bearing carbon atom. This hydrogen signal is shifted downfield of the other signals. Studies of many substituted cyclohexane rings have established that equatorial hydrogens generally appear about 0.5 ppm downfield of the corresponding axial hydrogens.
Background Note
The interconversion of cyclohexane chair conformations (“chair-flips”) typically occur so rapidly at room temperature that we cannot readily distinguish between the two conformations by NMR. why is that not the case here?
In this mixture of cis- and trans-4-tert-butylcyclohexanol, there is a signal at ~3.5 ppm for the methine hydrogen of the trans-isomer. Is this an axial or equatorial hydrogen? We also see a signal at ~4.0 ppm for the corresponding hydrogen of the cis-isomer. Is this an axial or equatorial hydrogen? The relative integration values for these two signals in the 1H NMR spectrum of your product correspond to the relative amounts of the two isomers in the product. Using these integrations, you will calculate the ratio of cis:trans diastereomers in your product.
Safety Considerations
4-tert-butylcyclohexanol and 4-tert-butycyclohexanone are irritants. Wear gloves and avoid all contact with skin, eyes, and clothing.
Sodium borohydride is harmful and all contact with skins or eyes should be avoided. Sodium borohydride is also water reactive; keep it away from water unless specifically instructed. If you spill solid NaBH4, wipe off as much as you can with a dry paper towel first, before using a slightly damp towel. Avoid contact of methanolic solutions of sodium borohydride with your skin, these solutions are highly caustic. If these solutions get on your skin, wash the affected area with a 1% acetic acid solution and then with copious amounts of running water for 15 minutes. Do not stopper flasks containing methanolic sodium borohydride. The solution slowly evolves hydrogen gas, and a dangerous buildup of pressure could occur in a stoppered flask.
The solution of L-Selectride is also flammable, corrosive, and highly reactive. It will be obtained using a syringe and only the TA should use the syringe to dispense this chemical; they will deliver it to your reaction vial. Follow all safe handling recommendations and recommendations for spills given in the SDS sheets for the other chemicals used in this experiment.
Procedure
L-Selectride Reduction
Add 1.94 mmol of 4-tert-butylcyclohexanone to a clean, dry vial and dissolve the ketone in 3 mL dry THF. Obtain a clean, dry 25-mL round bottom flask and equip it with a stir bar. Your TA will use a syringe to add 3.0 mL of a 1.0 M solution of L-Selectride to the flask. With care, transfer the solution of ketone to the reducing agent and stir the reaction solution for two hours.
After the two hours, add 1.5 mL of 80% ethanol to the reaction flask and stir the resulting solution for an additional 5 minutes. After this stirring period, add 1 mL of 6 M NaOH, followed by 1.2 mL of 30% H2O2. The addition of H2O2 must be done very carefully and dropwise. Transfer the reaction mixture to a 30-mL separatory funnel. Rinse the reaction flask with 3 mL Na2CO3 (sat., aq.) and transfer the sodium carbonate solution to the sep funnel. Separate the two phases. Extract the aqueous phase twice with diethyl ether (3 mL). Combine the organic phases in a small Erlenmeyer flask and dry over MgSO4. Filter and concentrate using a rotovap.
Sodium Borohydride Reduction
Add 3.24 mmol of 4-tert-butylcyclohexanone and a stir bar to a small Erlenmeyer flask. Dissolve the ketone in methanol to make a ~0.5 M solution. Ensure the ketone is fully dissolved before proceeding. If necessary, you may gently warm the mixture to facilitate dissolution. If you do this, the reaction flask should re-cool to room temperature before you move forward.
Working quickly (but carefully), measure 0.41 molar equiv. of sodium borohydride and immediately add this reagent to the solution of your ketone in one portion. Stir the reaction 20 minutes. After this time, add 2 mL of a 3 M solution of sulfuric acid to the reaction flask followed by 5 mL of water. Extract the product into two 8-10 mL portions of Et2O to extract the aqueous mixture. Wash the combined ether extracts sequentially with 5 mL of water and 5 mL of saturated sodium chloride. Dry the organic phase over MgSO4, filter into a tared round bottom flask and concentrate using a rotovap.
Product Analysis
Obtain a weight of each of your products and calculate the percent yields for both reduction reactions. Obtain a 1H NMR spectrum of the product mixture of both reactions. Set the integration of the smaller of these two signals to 1. The integrations of the signals at 4.02 and 3.52 ppm will enable you to determine the ratio of product isomers. Compare your values to those found in Chem. Educator 2009, 14, 232.
