10
The following protocol was developed by Dr. Lei Fang and PhD student Steven Hodge as part of an NSF grant. The purpose of this multi-step synthesis is to showcase two of the most important organic transformations in recent history. The efficiency in carbon–carbon and carbon–heteroatom bond formation that arose from these transformations have led to extensive growth in medicinal chemistry through heightened drug discovery. In fact, after amide bond formation, the Suzuki–Miyaura coupling reaction is the second most utilized reaction in medicinal chemistry as of 2014.1 Similarly, ring-closing metathesis offers synthetic chemists a robust platform to access unnatural macrocycles, which are found in a significant amount of pharmaceuticals.

In 2013, Dr. Lei Fang started his independent academic career as an assistant professor at Texas A&M University, where he leads a multidisciplinary research group studying functional organic materials with electronic, thermal or photo-activity. His current goal is to gain insights into design principles and structure–property relationships of these materials at both the molecular and the macroscopic scale by employing the toolboxes of synthetic chemistry and process engineering. In particular, the Fang research group focuses on the bottom-up synthesis and processing of ladder polymers and microporous polymer networks for applications associated with electronics and energy conversion/storage.

Steven Hodge received his bachelor’s degree in chemistry from Texas A&M University in 2021. He then joined the Fang group at Texas A&M as a PhD student that fall. Currently, he is a PhD candidate with his research focusing on helical polypyridine ligands. His research interest include pyridine-based ligands, conjugated polymers, and watching Aggie Football.
Learning Objectives
By this end of this two-week synthetic sequence, students will be able to…
- describe the challenges and key considerations of multi-step synthesis;
- describe the purpose and set-up of inert reaction conditions;
- safely and accurately use syringes to conduct organic reactions on mini- or microscale;
- describe the value and general process of in situ catalyst generation;
- dry-pack a compound for purification by flash column chromatography (full column or plug); and
- calculate reagent tables and yields for a multi-step synthesis.
Background
For most of our discipline’s history, introductory organic chemistry courses have been organized by functional group: Students learn organic chemistry by studying the names, structures, and basic preparation and reactivity of these organic motifs. As a result, the reactions taught are nearly always, well, old. For example, the Friedel–Crafts reaction was first reported in 1877, while the Grignard reaction was discovered in 1900. Though undoubtedly important, these classic organic reactions do not accurately represent the tools of modern synthetic organic chemistry labs.
Modern synthetic organic methods often rely on organometallic reagents, or compounds that possess a carbon–metal bond. These compounds induce unique reactivity and allow for powerful transformations, including the formation of carbon–carbon bonds. In this multi-step synthesis experiment, we will explore two Nobel Prize winning organometallic reactions over two weeks. The first in our sequence, the Suzuki (sometimes called Suzuki–Miyaura) reaction, uses palladium to effect a cross-coupling reaction. This reaction is highly scalable, relatively cheap, and can be applied to a wide range of substrates. As such, it is one of the most common reactions used in the production of pharmaceuticals and fine chemicals. In 2010, the importance of this reaction was recognized with a Nobel Prize awarded to Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki, the chemists responsible for the design and development this and similar palladium-catalyzed cross-coupling reactions.
The second reaction, ring-closing metathesis (RCM), is part of the larger family of olefin metathesis. Olefin metathesis describes the redistribution of alkene (olefin) fragments through the scission and regeneration of carbon–carbon double bonds. As the name suggests, ring-closing metathesis is an intramolecular variant that joins two terminal alkenes (tethered by a chain) to form an unsaturated ring. Olefin metathesis continues to expand its reach, with applications to polymers, pharmaceuticals, and other chemical industries. Yves Chauvin, Robert H. Grubbs and Richard R. Schrock were awarded the 2005 Nobel Prize in Chemistry for the development and mechanistic elucidation of this family of reactions.
Part 1. Suzuki–Miyaura Cross Coupling
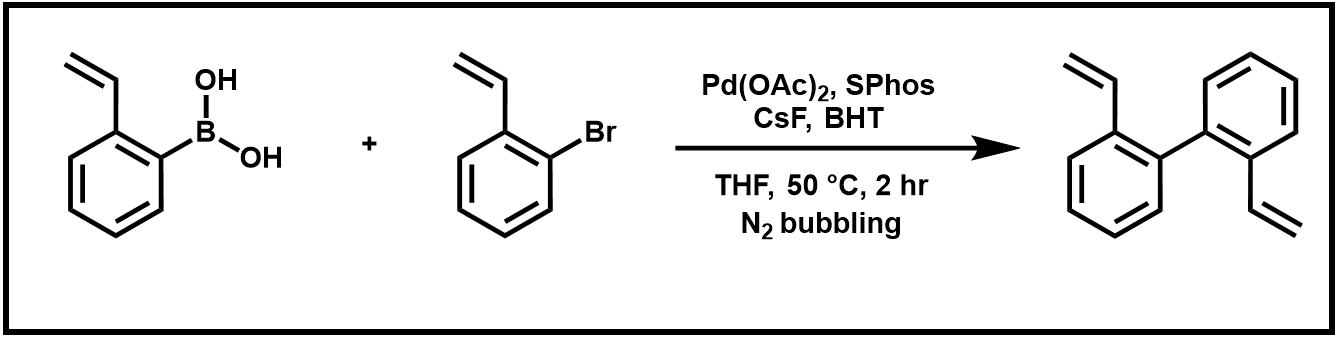
Over the next two weeks, we will word toward the two-step synthesis of phenanthrene. This sequence begins with a Suzuki coupling reaction of 2-vinyl boronic acid with 2-bromostyrene. The prepared biphenyl product then undergoes a ring-closing metathesis reaction using Grubbs’ “second generation” ruthenium catalyst.
Scheme 1. Suzuki Coupling Reaction of 2-vinylphenylboronic acid and and 2-bromostyrene
Experimental Protocol
Protocol Note
Palladium (0) catalysts are deactivated by oxygen. To help improve the reactivity of the catalyst, we are preparing it in the reaction flask (in situ). However, it’s imperative to limit the exposure of the reaction to air to maintain an active catalyst. The degassing step and bubbling of nitrogen gas through the reaction solution helps to remove atomospheric oxygen from the reaction solution/flask.
Reaction Setup
Using a small crystallizing dish, prepare a 50 °C water bath.
Equip a 25-mL round bottom flask with a stir bar. Using a syringe, add 1.25 mmol of 2-bromostyrene to the flask. The entire class will use the same syringe to obtain this reagent.
Then, add 1.2 molar equiv. of 2-vinylphenylboronic acid, 0.2 molar equiv. SPhos, and 5 molar equiv. of CsF. Finally, add 1–2 small crystals of butylated hydroxytoluene, BHT. Dissolve the mixture in 7.5 mL THF (not all of the CsF will dissolve) and stopper the flask with a rubber septum. Insert two needles into the septum of your reaction flask. The longer needle will be your nitrogen inlet and will be connected to a balloon filled with nitrogen. The shorter needle should be open to the air. The longer needle should be submerged in the reaction solution. The positive pressure of the nitrogen balloon will degas the solution by bubbling nitrogen through the reaction solution. While stirring your reaction solution, degas the solution this way for 10 minutes. Refill the balloon with nitrogen if/as necessary.
Remove the needles, stop the stir bar and then remove the septum, then quickly add 0.1 molar equiv. of Pd(OAc)2 to the reaction flask. Immediately replace the septum and needle as before, resume stirring and bubble nitrogen through the reaction for an additional 7–8 minutes.
After degassing the reaction, remove the outlet needle and heat the reaction flask in the water bath and heat the reaction to 50 C while stirring for 2 hours (maintaining the setup under nitrogen). After this time, keep the septum in place and place the reaction flask inside a beaker in your drawer to ensure the flask does not tip over. The reaction will continue at room temperature until the next lab period.
Purification
At the start of the next lab period, remove the stir bar and then add approximately 1–2 g dry silica gel powder. Remove the THF by rotary evaporation to absorb your product on the dry silica. The product will be purified by running it through a short plug of silica, but since dissolving the product in hexanes is challenging, using the pre-absorbed method helps ensure the product is added to the column as a narrow band. The main objective is to separate the product from the palladium, which inhibits the next step of the reaction.
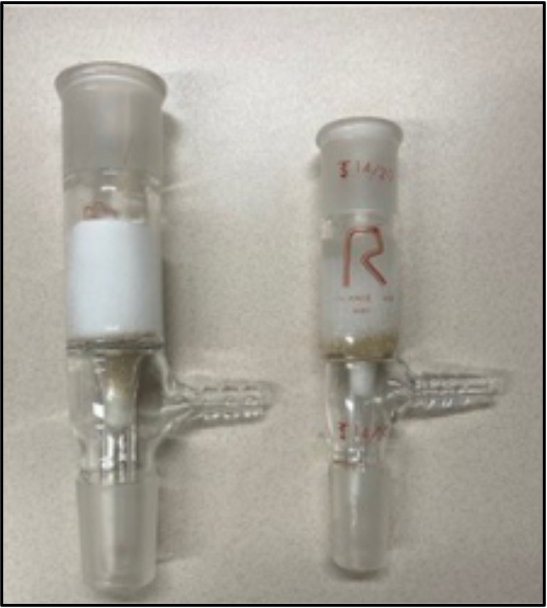
Figure 1. Silica plug in an inlet adapter. Cotton is pushed into the narrow vacuum tube, topped with sand and then silica.
Prepare a plug of silica using an inlet adapter as shown in Figure 1. Plug the narrow vacuum stem with a small amount of cotton, cover this with a layer of sand and then 2–3 cm dry silica. Condition the silica with hexanes and gently tap the sides of the inlet adapter to remove any air bubbles; let the solvent drain through by gravity.
Pour the dry silica powder with your product adsorbed onto it carefully onto the top of the silica plug. Use small portions (0.5–1 mL) of hexanes to wash the solids if necessary and add this solution to the top of the silica plug. Collect the product in small, 2–3 mL, fractions. Check each fraction for the presence of the product by spotting a small amount on a TLC plate. The fractions with nothing present can be discarded. Continue collecting fractions until you have a fraction with no material visible on a TLC plate under UV light.
Analyze your fractions containing product by TLC (elute with hexanes).
Protocol Note
If present, unreacted bromostyrene will elute first, followed by the coupled product and then BHT.
Combine all fractions containing product into a tared round-bottom flask and concentrate them under reduced pressure. The coupled product is a solid when pure and very dry, but impurities will make it very difficult to solidify. After removing the solvents, although the product should be a solid, there is a chance it will not be if it is contaminated with bromostyrene and possible also some solvent, neither of which affect the future ring closing metathesis reaction.
Characterization
Characterize your product by 1H NMR spectroscopy. Calculate a yield and save the rest of your product for the metathesis reaction.
Safety
Use caution when handling needles. Used needles should be put into the sharps container in the hood. DO NOT recap used needles.
When preparing your silica plug, use a wooden or metal (not glass) stick to push the cotton down into the vacuum stem of the inlet adapter. Always handle the silica in a hood.
Waste Disposal
All aqueous solutions should be collected in a container specially marked for aqueous wastes. Place organic liquids in the container designated for nonhalogenated organic waste unless they contain dichloromethane. Waste solutions that contain dichloromethane should be placed in halogenated organic waste.
Used silica and TLC plates should be placed in the solid waste containers in the hood.
END OF WEEK ONE
Part 2. Ring-Closing Metathesis
Last week, you ran a Suzuki–Miyaura Reaction to produce your biphenyl product (Scheme 1).
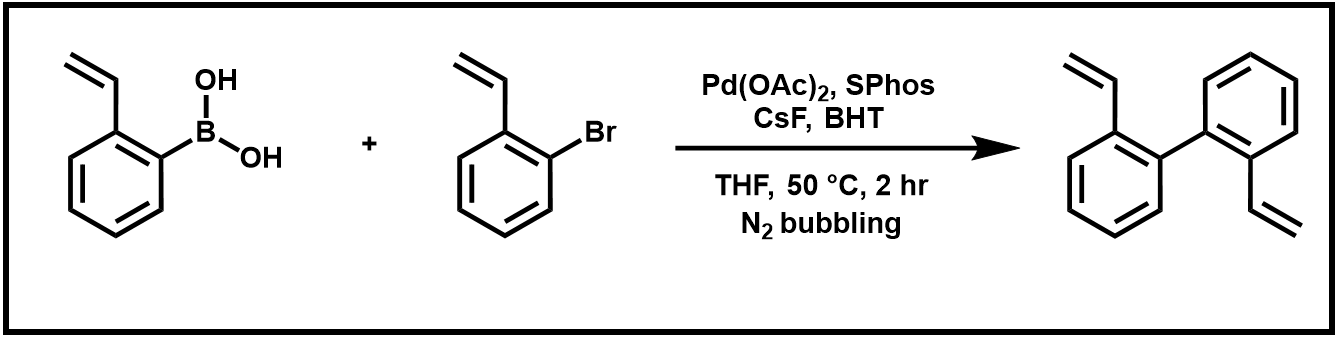 Scheme 1. Suzuki coupling reaction of 2-vinyl boronic acid and 2-bromostyrene.
Scheme 1. Suzuki coupling reaction of 2-vinyl boronic acid and 2-bromostyrene.
This week, you will use your biphenyl product to run a ring-closing metathesis reaction using Grubbs’ “second generation” ruthenium catalyst to produce phenanthrene.

Scheme 2. Ring-closing metathesis of 2,2′-divinylbiphenyl
Experimental Protocol
Reaction Setup
Using your small crystallizing dish, prepare a 35 ºC water bath.
To a 25-mL round bottom flask equipped with a stir bar, add your 2,2’-divinylbiphenyl product from the Suzuki coupling reaction. Add a crystal of butylated hydroxytoluene (BHT). Add 0.03 molar equiv. of the Grubs II catalyst (FW: 848.97 g/mol).
Protocol Note
You must scale the amount of catalyst added based on the yield of your previous reaction.
Add about 3 mL dichloromethane (do not ue less than 2 mL of solvent for this reaction) and seal the round bottom flask with a septum. Pierce the septum with a short needle that is open to the air. Place the reaction in a 35 ºC water bath and stir for two hours. Then, remove the needle and leave the reaction in your drawer until the next lab period.
Purification
Prepare a silica plug as you did for the previous step of the reaction and run the crude reaction solution through the silica plug using dichloromethane. The phenanthrene product essentially runs with the solvent front in dichloromethane. The separation should be monitored by TLC.
Collect all the fractions containing your phenanthrene product and concentrated under reduced pressure (i.e., using a rotavap). The residual solid should be precipitated from a water/ethanol mixed solvent system.
To do this, dissolve the crude product in the minimum amount of ethanol (no require more than ~5 mL). Once everything is dissolved, add water dropwise until the product has fully precipitated from solution.
Protocol Note
If you are having difficulty in fully dissolving the crude product in ethanol, gently heat the solution until it is fully dissolved.
Cool the flask in an ice–water bath. Collect the product by vacuum filtration; rinse the product with water.
Characterization
Characterize your product by 1H NMR, IR, and melting point. Calculate the percent yield for the full reaction sequence.
Safety & Waste Disposal
Be careful when handling needles; used needles should be disposed of in the fume hood sharps container. Never recap used needles.
After collecting your product, discard the filtrate in the organic waste bottle.
References
Buskes, M.J.; Blanco, M.-J. Impact of Cross-Coupling Reactions in Drug Discovery and Development. Molecules 2020, 25, 3493.
A cross-coupling reaction in organic synthesis occurs when two fragments are joined together with the aid of a metal catalyst.